Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Genistein Preserves Pancreatic β-Cells Against H2O2-Induced Apoptosis in Mice Insulinoma Cell Lines
*Corresponding author: Rahman M Hafizur, Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Pakistan.
Received:May 21, 2021; Published: July 29, 2022
DOI: 10.34297/AJBSR.2022.16.002286
Abstract
The plant-based isoflavones, particularly genistein, have been shown as a potent stimulator of insulin secretion with promising hypoglycemic activity; however, very little is known about its anti-apoptotic potential. The current pre-clinical investigations relate genistein as a β-cell protective agent, using in vitro model of H2O2-induced apoptosis in mouse insulinoma (MIN6) cells. Apoptotic induction was examined by annexin-V/propidium iodide staining, cleaved casp3 through immunostaining followed by fluorescent microscopy, whereas expressions of caspase 3 and caspase 9 genes by RT-PCR. We found that in genistein pre-treated conditions, few cells fall in the early and late stage of apoptosis as compared to H2O2-treatment only. In subsequent GS-treated MIN6 cells, almost no detectable levels of cleaved casp-3, an oxidative stress-mediated activator, confirmed the anti-apoptotic effect mediated by genistein. We observed a significantly lower level of casp-9; however, no effect on casp-3 expression in cells pre-treated with genistein. Moreover, genistein exhibited no cytotoxic effect at an effective concentration. The pre-clinical data revealed that genistein preserves pancreatic β-cells against H2O2-induced apoptosis in MIN6 cells and appeared suitable for further investigations.
Keywords: Genistein, Apoptosis, Caspases, β-cell protection
Introduction
During chronic hyperglycemic conditions, pancreatic β-cells become exhausted through numerous defects, particularly impaired signaling pathways of insulin secretion and β-cell apoptosis [1]. There are accumulative pieces of evidence that relate β-cell apoptosis to decreased β-cell mass and trigger diabetes progression [2-4]. According to these studies, apoptosis is complex processes triggered by the results of either intrinsic or extrinsic signal in the activation of specific enzyme pro-caspases, which on cleavage; undergo structural changes and converts to active caspases. Activation of procaspases results in apoptosis. These caspases may be the initiator caspases (8, 9, and 10) that initiate the event of the caspase induced cell death and ultimately activate the executioner caspases (3, 6, and 7). Executioner caspases cleave specific substrates, which result in the characteristic changes of apoptosis. In addition, mitochondria releasing apoptosis-inducing factor (apaf-1) and pro-caspase 9 can form a complex with the cytochrome c, which is also released from the mitochondria due to the formation of the pores. This complex is called the apoptozole, which activates caspase 3, an executioner caspase, resulting in apoptosis.
Recently, few drugs are available with anti-apoptotic and insulin secretory effects on pancreatic β-cells and are more effective than the classical drugs having a glucose-lowering effect. Some examples of such drugs include GLP-1 analogs and DPP-IV inhibitors. Long-acting GLP-1 analogs such as exendin-IV improve the pancreatic β-cell functions by producing different effects, which includes the inhibition of apoptosis, as well as insulin secretory potential, the activity not found in most of the classical drugs used for the treatment of diabetes [5,6]. The natural compounds have been the focus of alternative therapeutic strategies not only for combating the disease, but also a biocompatible natural entity in patients against diabetes. Therefore, there is a strong urge to explore lead compounds, particularly from natural sources that along with enhanced insulin dynamic may also protect β-cells for better treatment against diabetes. The plant-based isoflavones, particularly genistein, have been shown to exhibit strong anti-diabetic potential and related metabolic disorders in numerous pre-clinical research exertions [7,8]. Chemically genistein is one of the most prevalent isoflavones originally purified from plant source Genista tinctoria L. that later has been reported mainly in soy- and legume-based sources with typical examples of soybeans and lupins, respectively [9]. Biochemically, genistein has been synthesized to fulfill its ever-growing demand by several strategic approaches such as through correspondent ketones cyclization, using microwave oven method, enzyme-based production via fermentation of bioengineered yeast cells of Saccharomyces cerevisiae, and enhanced transformation of genistein by targeting the correspondent phenylpropanoid pathway [9].
There are several preclinical studies, which have been reported genistein (GS), a potent stimulator of insulin secretion [8,10] with promising hypoglycemic potential [11,12] however, its antiapoptotic potential is still needed to be investigated. In the current study, we hypothesize that genistein may have effects against apoptosis in pancreatic beta cells and exert an overall effect against diabetes. To test this hypothesis, we evaluated the anti-apoptotic potential mediated by genistein-treatments, using a working model of apoptosis-induced mouse insulinoma (MIN6) cells, exposed to higher H2O2 concentration (300μM). Subsequently, we performed genetic analysis such as real-time PCR study of mRNA expression to evaluate the effect of genistein on the modulation of apoptotic genes namely, caspase 3 and caspase 9, respectively. We found that genistein decreases apoptosis and preserves pancreatic β-cells. Additionally, MIN6 cells treated with genistein were found with no detectable level of cleaved caspase 3 supported by genetic analysis confirms that genistein may be one the lead compounds with the dual activity of insulin secretion and protection against apoptosis.
Materials and Method
Reagents
DMEM high glucose media and trypsin-EDTA were purchased from Thermos Fischer Scientific (Waltham, MA, USA); fetal bovine serum (FBS) and 4’,6-Diamidino-2-phenylindole (DAPI) from Sigma (St. Louis, MO, USA); cleaved caspase 3 from cell signaling (Danvers, MA, USA); secondary antibodies of Cy2-goat anti-rabbit IgG and Alexa 594-donkey anti-mouse IgG were purchased from Jackson Immuno research (Baltimore, PA, USA); Annexin-V-FITC from Invitrogen (Carlsbad, CA, USA).
Cell Culture
In vitro experiments were carried out in MIN6 cell lines kindly provided by Dr. Jun-ichi-Miyazaki of Osaka University, Japan. Cells were cultured in DMEM containing 25 mM glucose supplemented with 12% FBS, 2mM glutamine, 100U/ml penicillin, 100μg/ml streptomycin, and 5μl/l β-mercaptoethanol at 37℃ in a humidified atmosphere of 5% CO2 / 95% air.
Immunostaining of Pancreatic β-Cells
For immunostaining, cells were seeded onto 12 mm diameter round coverslips placed in a 24 well plate. After experimental procedures, cells were washed with phosphate-buffered saline 0.01M, pH 7.4 (PBS) and fixed with 2% paraformaldehyde (20 min, ambient temperature). Fixed cells were washed and permeabilized with 0.2% Triton X-100 for 10 min. Cells were blocked with 2% serum (in which the secondary antibody is raised) for 10 min and were incubated with appropriate primary antibody dilutions in serum for 90 min at 42℃. At the end of primary antibody incubation, cells were washed with PBS and incubated with secondary antibody for 60 min at 42℃. Cells were stained for DAPI (0.5-1μg/ml) for 2 min. After washing the cells, coverslips were removed from the 24 well plate and mounted upside down over the glass slide having mounting media and visualized under the microscope.
Microscopy and Image Processing
Nikon 90i fluorescent microscope (Japan) was used for microscopy and DXM 1200C camera was used for image acquisition through NIS-Elements software AR 3.0. Minimal processing was done via the image processing software adobe photoshop CS2 for the selected images. In the case of the fluorescence intensity measurement, the pinhole setting was kept constant during capture and little to no image processing was done.
Real-Time PCR Analysis
RNA isolation:After completion of treatments on MIN6 cells, total RNA was isolated from MIN6 cells by using TRIzol (Invitrogen, NY, USA) reagent following the manufacturer’s instruction. Following RNA isolation, the concentration and purity of RNA were determined using nanodrop by taking their absorbance at 260 nm and 260/280 ratio. The isolated RNA was stored at -80℃ until further analysis.
cDNA Synthesis:After isolation of RNA, cDNA was synthesized from 1μg of RNA using Revert Aid First strand cDNA synthesis Kit (Thermo Scientific, MA, USA) as mentioned in the kit protocol.
RT-PCR Analysis:Following cDNA synthesis, real-time PCR of the specified genes was performed using Real-time PCR instrument (Stratagene Mx3000p, SC, USA). SYBR green master mix (Fermentas, Burlington, Canada) was used for RT-PCR reactions using appropriate primers designed for the genes (Table), and a reaction mixture of 20μL was made. The genes were amplified using 40 cycles at 95℃ for 30s, 60℃ for 30s, and 72℃ for 30s, followed by 5 min final extension at 72℃. The transcription level of GAPDH was used as a control with the same amount of RNA and thermal cycles (Table).
Statistical Analysis
All statistical analyses were performed by using the SPSS (Statistical Package for Social Sciences) package for Windows version 11.0 (SPSS, Inc., Chicago, IL, USA). All values are expressed as mean ± SEM. To compare data between and within groups, unpaired and paired t-tests (2-tailed) were performed. The significance of differences among the groups’ mean values was calculated by one-way ANOVA with Bonferroni post hoc tests. p<0.05 were considered as statistically significant.
Results and Discussion
Genistein Decreases Oxidative Stress Mediated Apoptosis in MIN6 Cells
Oxidative stress plays critical roles in the pathophysiology and impaired cell signalosome. Particularly, in pancreatic β-cells, the level of antioxidant enzymes is much lower than that of liver e.g., superoxide dismutase expression is less than half in case of pancreatic islets in comparison to liver. Moreover, the glutathione peroxidase expression is just limited to 15% and it is even harder to detect the levels of catalase [13]. This lower antioxidant defense system along with constant increase in the concentration of H2O2 exposes the β-cells to oxidative stress-induced damages. We investigated whether pre-treatment of genistein has a protective effect against oxidative stress and cell death mediated by H2O2 in the pancreatic cells. Pre-treated MIN 6 cells with genistein were exposed to oxidative stress with H2O2 and apoptotic cells were detected via Annexin-V/Propidium Iodide (AV/PI) staining. We observed that few cells fall in early (i.e., AV +ve) and late-stage (AV +ve / PI +ve) of apoptosis as compared to that of H2O2-treatment only. Quantification data showed a significant decrease in Annexin V-FITC, and PI-positive cells by genistein treatment (Figure 1A, Figure 1B). The data suggest that genistein exhibited significant protection of MIN6 cells against H2O2 induced apoptosis.
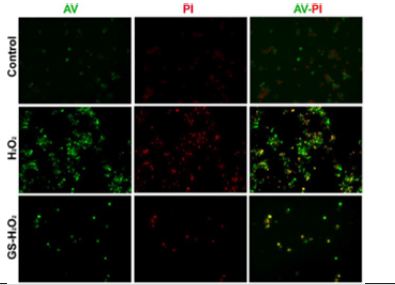
Figure 1A: Genistein (GS) prevents apoptosis in MIN6 cells. Note*: Representative images of, Annexin V-FITC and PI double fluorescence staining showing protection of MIN6 cell apoptosis after H2O2 treatment by GS. Annexin V and PI were visualized by green signal and red signals, respectively. Magnification × 200.
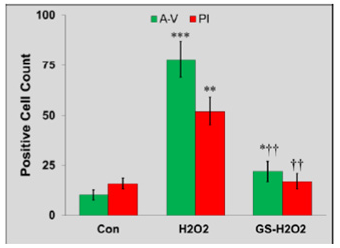
Figure 1B: Genistein (GS) prevents apoptosis in MIN6 cells. Note*: The representative quantification data shows a significant increase in Annexin V-FITC, and PI-positive cells by H2O2 treatment (*P<0.05, **P<0.01, ***P<0.001 vs. Control). GS pre-treatment significantly decreased Annexin V-FITC, and PI-positive cells (†P<0.05, ††P<0.01 vs. H2O2).
Genistein Modulates Cleavage of Caspase-3 Protein and Expression of Caspase-9 Genes to Inhibit Apoptosis
As detection of cleaved caspase 3 activates caspase 3, which mediates nuclear fragmentation and DNA damage eventually leading to cell death by relaying apoptosis signals [14]. Next, we examine whether genistein does decrease the expression of cleaved caspase 3 in MIN 6 cells. We noted that in GS-treated MIN6 cells almost no detectable levels of cleaved casp3 (an indicator of the ongoing apoptotic process) were observed after 24 hours as compared to that of H2O2 treatment alone (Figure 2A). These data support that genistein decreases the mitochondrial-mediated apoptotic process.
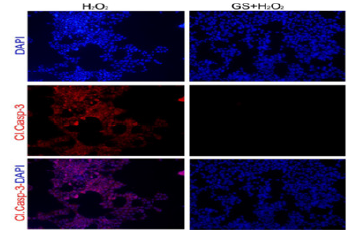
Figure 2A: Genistein, GS abolishes the expression of cleaved casp3 and pro-caspase genes in MIN6 cells. A. Note*: GS lowers casp3 level (indicated by red staining), exposed to H2O2. DAPI (indicated by blue staining) was performed for staining the nuclei of the cells. Magnification × 200.
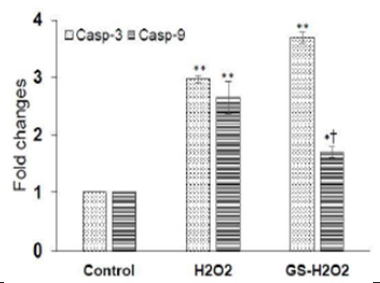
Figure 2B: Genistein, GS abolishes the expression of cleaved casp3 and pro-caspase genes in MIN6 cells. Note*: GS significantly reduces the expression of casp9 genes increased by H2O2; however, no significant effect of GS on casp3 was observed. Values are mean ± S.E.M. for 3 different experiments. *P<0.01, **P<0.001 vs. Control. †P<0.01, vs. H2O2.
In a subsequent experiment, we performed the gene expression analysis through real-time PCR to evaluate the effect of genistein on the modulation of apoptotic genes namely, caspase 3 and caspase 9. Caspase 3 and caspase 9 play a pivotal role in apoptosis, the former being an executioner caspase, whereas the latter is thought as a crucial contributor to apoptozole formation [15,16]. We found that H2O2 significantly increased the expression of both casp-3 (2.969 ± 0.064 vs. 1.00; P<0.001) and casp-9 gene (2.647 ± 0.285 vs. 1.00; P<0.001) as compared to that of control cells. The pre-treatment with genistein significantly lowers the levels of casp9 (1.69 ± 0.103 vs. 2.969 ± 0.064; P<0.001); interestingly, no effect was found on casp-3 gene expression (3.69 ± 0.09 vs. 2.969 ± 0.064; P>0.05) in comparison to H2O2 treated cells (Figure 2B). The mRNA expression data suggest that GS downregulated the pro-apoptotic protein gene for caspase 9; however, does not affect caspase 3.
From these observations, we can deduce that genistein decreases β-cell apoptosis through maintaining mitochondrial membrane potential, decreasing the expression of caspase 9 protein, and affected the cleavage of caspase 3. These findings are in good agreement with previous work, where genistein was found to protect DNA damage exposed to H2O2 [17], cytoprotective activity [18], beta-cell proliferation activity [8], and cardioprotective activity, respectively [9]. Taken together, these pre-clinical investigations concluded that genistein exerts dual anti-diabetic activities as insulin secretagogue and anti-apoptotic agent in the pancreatic beta cells.
Conflict of Interest
No Conflict of interest.
Acknowledgments
This work was supported by a grant [no. HEC/R&D/ NRPU/2017/8544] to Md. Hafizur Rahman from the Higher Education Commission (HEC), Pakistan.
References
- Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116(7): 1802-1812.
- Lee SC, Pervaiz S (2007) Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol 39(3): 497-504.
- Simone F, Adrienne M Gorman, Osamu H, Samali A (2010) Cellular stress responses: cell survival and cell death. Int J Cell Biol 23.
- Daniel N, Korsmeyer S (2004) Cell death: Critical control points. Cell 116(2): 205-219.
- Nielsen LL, Young AA, Parkes DG (2004) Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 117(2): 77-88.
- Deacon CF, Ahrén B, Holst JJ (2004) Inhibitors of dipeptidyl peptidase IV: a novel approach for the prevention and treatment of type 2 diabetes? Expert Opin Investig Drugs 13(9): 1091-1102.
- Fu Z, Zhang W, Zhen W, Lum H, Nadler J, et al. (2010) Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology 151(7): 3026-3037.
- Liu D, Zhen W, Yang Z, Carter JD, Si H, et al. (2006) Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes 55(4): 1043-1050.
- Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, et al. (2015) Genistein and cancer: current status, challenges, and future directions. Adv Nutr 6(4): 408-419.
- Fu Z, Liu D (2009) Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur J Pharmacol 616(1-3): 321-327.
- Fu Z, Gilbert E R, Pfeiffer L, Zhang Y, Fu Y, et al. (2012) Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl Physiol Nutr Metab 37(3): 480-488.
- Behloul N, Wu G (2013) Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol 698(1-3) 31-38.
- Lenzen S, Drinkgern J, Tiedge M (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20(3): 463-466.
- Suhaili S, Karimian H, Stellato M, Lee T, Aguilar M (2017) Mitochondrial outer membrane permeabilization: a focus on the role of mitochondrial membrane structural organization. Biophys Rev 9(4): 443-457.
- Bock FJ, Tait SW (2020) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21(2): 85-100.
- Zampieri C, Ganini C, Melino G (2020) The biochemistry of cell death.
- Raschke M, Rowland IR, Magee PJ, Pool Zobel BL (2006) Genistein protects prostate cells against hydrogen peroxide-induced DNA damage and induces the expression of genes involved in the defense against oxidative stress. Carcinogenesis 27(11): 2322-2330.
- Gilbert ER, Liu D (2013) Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food & function 4(2): 200-212.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.